Abstract
Introduction
Ibrutinib, a first-in-class, oral, covalent inhibitor of Bruton's tyrosine kinase, is approved in many countries for treatment-naïve (TN) and previously treated CLL, supported by improved response rates, progression-free survival (PFS), and overall survival (OS) vs chlorambucil in RESONATE-2™ (NCT01722487; Burger JA, et al. N Engl J Med. 2015;373:2425-2437) and ofatumumab in RESONATE™ (NCT01578707; Byrd JC, et al. N Engl J Med. 2014;371:213-223). We analyzed survival outcomes for ibrutinib vs RW treatments for TN and relapsed/refractory (R/R) CLL by del11q status, an adverse prognostic factor linked with poor patient outcomes despite conventional chemoimmunotherapy. An adjusted comparison restricted to patients with confirmed del11q status was conducted using patient-level data from RESONATE-2™ and RESONATE™ and RW databases from 2 countries.
Methods
The Lyon-Sud RW database holds medical records for CLL patients diagnosed between 1980 and 2017 from the Centre Hospitalier Lyon-Sud, France; the Chronic Lymphocytic Leukemia Registry (CLLEAR) RW database holds medical records for CLL patients diagnosed between 1988 and 2017 from 7 academic centers in the Czech Republic. TN CLL database patients were selected using RESONATE-2™ criteria (which excluded those aged < 65 years or del17p positive). For the R/R group, patients ≥ 18 years of age or those with del17p were allowed per RESONATE™ inclusion criteria. PFS and OS outcomes by del11q status were compared between the ibrutinib arms of RESONATE-2™/RESONATE™ and physicians' choice (PC) treatment in the pooled RW databases (excluding ibrutinib). A multivariate Cox proportional hazards model was fitted on pooled RCT/RW data to estimate adjusted hazard ratios (HRs) for effect of ibrutinib vs RW PC treatment using age, sex, and treatment line as covariates. The unit of observation for the RW databases was the treatment line (rather than patient) number; RW patients receiving multiple lines of therapy contributed to multiple observations, and baseline was defined as the line-specific treatment start date.
Results
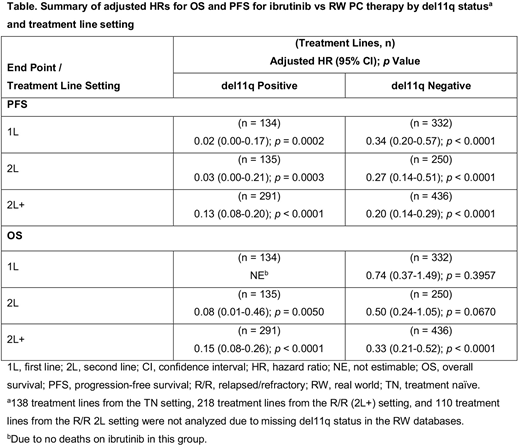
For the RW TN cohort, 466 treatment lines were analyzed; 134 (28.8%) patients were del11q positive. In RESONATE-2™, 29/136 (21.3%) TN patients were del11q positive. The table below shows adjusted HRs for PFS and OS for patients with/without del11q. For the del11q-positive subgroup, adjusted HRs for ibrutinib vs RW PC first-line (1L) therapy were 0.02 (95% confidence interval [CI], 0.00-0.17; p = 0.0002) for PFS and not estimable for OS (no deaths on ibrutinib). For the del11q-negative subgroup, adjusted HRs were 0.34 (95% CI, 0.2-0.57; p < 0.0001) for PFS and 0.74 (95% CI, 0.37-1.49; p = 0.3957) for OS. For second-line (2L) PC treatment in the RW R/R cohort, 385 treatment lines were analyzed; 135 (35.1%) were from del11q positive patients. In RESONATE™, 13 (37.1%) 2L patients were del11q positive. For the del11q-positive subgroup, adjusted HRs for ibrutinib vs RW PC of 2L therapy were 0.03 (0.00-0.21; p = 0.0003) for PFS and 0.08 (0.01-0.46; p = 0.0050) for OS. For the del11q-negative subgroup, adjusted HRs were 0.27 (0.14-0.51; p < 0.0001) for PFS and 0.50 (0.24-1.05; p = 0.0670) for OS. For 2L and beyond (2L+) treatment in the RW R/R cohort, 727 treatment lines were analyzed; 291 (40.0%) were from del11q positive patients. In RESONATE™, 63/195 (32.3%) 2L+ patients were del11q positive. For the del11q-positive subgroup, adjusted HRs (95% CIs) for ibrutinib vs RW PC of 2L+ therapy were 0.13 (0.08-0.20; p < 0.0001) for PFS and 0.15 (0.08-0.26; p < 0.0001) for OS. For the del11q-negative subgroup, adjusted HRs were 0.20 (0.14-0.29; p < 0.0001) for PFS and 0.33 (0.21-0.52; p < 0.0001) for OS.
Conclusions
Adjusted comparisons of the registration trial (RESONATE-2™/RESONATE™) and RW patient-level data suggest that OS and PFS improvements with ibrutinib vs PC therapies are pronounced for patients with CLL harboring del11q compared with those who are del11q negative. The findings inform physicians of the comparative effectiveness of ibrutinib in the RW for patients with this high-risk cytogenetic abnormality.
Funding Source:
This project was sponsored by Janssen Pharmaceutica NV, and Pharmacyclics LLC, an AbbVie Company. The real-world databases are independently owned. Writing assistance was provided by Emma Fulkes of PAREXEL and funded by Janssen Pharmaceutica NV.
Salles:Takeda: Honoraria; Acerta: Honoraria; Epizyme: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; AbbVie: Honoraria; Gilead: Honoraria; Roche: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Servier: Honoraria; Janssen: Honoraria; Merck: Honoraria; Morphosys: Honoraria. Besson:Janssen Pharmaceutica NV: Employment. Doyle:Janssen Pharmaceutica NV: Employment. Garside:Janssen Pharmaceutica NV: Employment. Spacek:Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria. Doubek:Novartis: Consultancy; AbbVie: Consultancy, Research Funding; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Affimed: Research Funding; Gilead: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.